Tips for Preparing Your Carbon (13C) and Nitrogen (15N) Solid Samples
|
Do...
|
Don't...
|
| - Do crimp samples into a compact spherical, cylindrical, or cubic shape, with maximum dimensions of 5.5 mm for 5x9 mm tins (or 8 mm for 9x10 mm tins) - Make sure tin capsule openings are folded over more than once if you can't compress the samples - Place an index card over the tray before securing the cover if you have small samples - Use clean equipment to handle the samples and tins. |
- Don't shape your samples into very flat disks (<1 mm) or thin tube/cigar shapes. - Don't ship capsules that only have their openings pinched closed or folded once. - Don't ship samples that are leaking. - Don't over-fill capsules. Excess filter paper can be trimmed off to reduce volume. - Don't contaminate samples by handling with bare hands or by using sandpaper to grind plant or wood samples. |
 |
 |
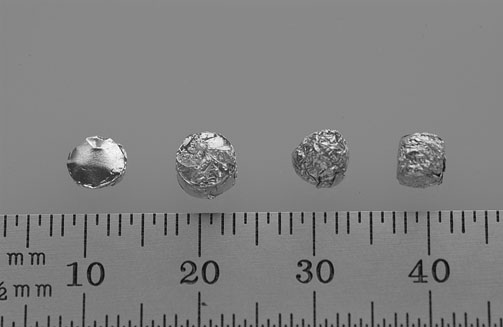
| Above are examples of standard sized samples. From left to right: ~1 mm tall X 5.5 mm diameter cylindrical sample ~ 4 mm tall X 5.5 mm diameter cylindrical sample ~5 mm spherical sample ~5 mm cubical sample |
Above are examples of improperly shaped samples. From left to right: - Tube/cigar shaped sample >5 mm long. - Very flat, flake- shaped sample, less than 1 mm tall and over 5.5 mm wide. - The opening of this capsule has only been pinched closed. - The opening of this capsule has only been folded over one time. |
 |
 |
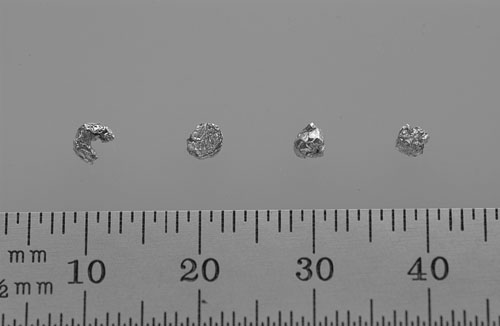
| Above are examples of very small and compact samples which can slip between the gap that is manufactured into all 96-well trays. Use an index card to cover the tray before taping on the cover. From left to right: ~1 mm tall X 3 mm wide crescent shaped sample ~1 mm tall X 3 mm wide cylindrical sample ~3 mm spherical sample ~2.5 mm cubical sample. |
Above are examples of very large samples. Usually G/F filters From left to right: ~6 mm tall X 5 mm wide, this is an example of a "good" large sample. ~6 mm tall X 6 mm wide, this sample is too wide for transport in a 96-well plate; this sample should be organized in a 48 or 24 well plate. ~12 mm tall X 5.5 mm wide. While this sample is narrow enough, the height of the sample will cause the auto-sampler to chop off the top portion, therefore contaminating later samples and/or clogging the machine. -Over-stuffed with filter, this sample has burst. This may occur as you are closing your samples, or later during shipping as filter tends to expand after being compressed. Trimming off excess filter will reduce the volume of filter paper being packaged. |
How to encapsulate samples
Please encapsulate solid samples in tin (Sn) capsules so they remain intact and do not leak and contaminate other samples during shipping. The tin is an important combustion catalyst. While the use of silver (Ag) capsules may be necessary for acid fumed materials, the use of any alternative capsule (e.g. nickel (Ni) capsules) is unacceptable.
Test your crimping-sealing method using a dummy sample in a tray. Shake and flip the tray to mimic agitation during shipping to see if the sample stays in its well or leaks from the tin. Very small or flat samples can escape their wells due to the gaps created by evaporation rings on 96, 48, and 24-well trays. Do not underestimate the gap between the tray and cover! This is the number one cause of lost samples. Fill the gap by placing and index card and/or foam sheet between the tray and lid. Do not use Parafilm, tissue, or adhesive tape to cover the wells - this can contaminate your samples.
You can make a crimper plate (pictured below) to hold tins and use a metal dowel to compress them in the plate holes. Once compressed, the closed samples can be transferred directly into a 96-well tray.
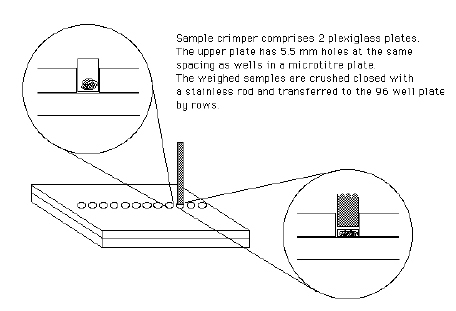
Alternatively, you can manually seal the tins using two sets of forceps with blunt tips to form them into cubical or cylindrical shapes. First, pinch the top closed and fold it over. Then, press and hold the capsule top to bottom while using the other forceps to pinch the sides inward. Repeat until you have a compact tightly packed tin.
Visit the SIF for encapsulation training
The SIF has occasional availability to train visitors on how to encapsulate their samples. Training takes about 30 minutes. UC Davis researchers and campus affiliates may request permission to use our guest balance by appointment. Check our Guest Balance Calendar for availability.
Avoid contaminating your samples with packing materials
-Do not wrap or cushion your individual samples with Kimwipe tissue or cotton wool when placing samples in a 96-well plate. Tissue and cotton particles may contribute C&N.
-Do not mark your samples with ink or pencil for ID purposes. Ink and pencil may contribute C&N.
-Do not place loose dessicant in direct contact with your 96-well plate. The resulting dust will contaminate your samples. Dessicant should be self-contained and separate from your 96-well plate.
-Do not place adhesive tape or Parafilm directly over wells in a 96-well plate. The adhesive will transfer onto your samples causing them to stick to our autosampler and will result in sample loss. If you must close the gap between the 96-well plate and cover, trim an index card(s) to size and place the index card(s) between the plate and cover.
Use KHSO4 for ammonia diffusion traps
Use KHSO4, rather than H2SO4, on the ammonia trapping disk to avoid rapid corrosion of the tin (Sn) capsule. Please do not use silver (Ag) capsules with KHSO4 ammonia traps, otherwise your samples will be charged for "difficult combustion" due to silver capsules. Adjust the volume of extract to obtain optimal mass of N on the disk, ideally 100 µg N in natural abundance samples, 20 - 50 µg N in enriched samples.
Use large tins for bulky samples
Large 9x10 mm tin capsules are helpful for encapsulating bulky items, like filter disks, which must be tightly packaged to maintain a compact form (no larger than 8 mm wide X 8 mm tall) in the autosampler. If samples are too large for a 96-well tray, ship them in a 24 or 48-well tray instead. Please do not force large samples into a 96-well tray, they will expand during shipping and we will not be able to extract these samples from their wells.
Keep enriched samples separate from natural abundance samples
For enriched / tracer experiment samples, arrange samples from low to high enrichment to avoid wide fluctuations in isotope content. Place non-enriched control samples ahead of enriched samples within the same tray. Use separate trays for different enriched and natural abundance experiments to avoid contamination. Optimize your enriched sample weights for 20 µg N in the sample, with a maximum of 100 µg N (on the Sample Weight Calculator, use the "Smallest sample weight" to "Optimal sample weight" as your target range).
Include replicates to check precision, especially for complex matrices
The SIF runs calibrated standards to confirm the precision of our analyses. Client replicates are recommended to check the precision of your samples, especially for samples that exceed our detection limits. The number of replicates you provide is at the researcher’s discretion as we understand that replicates incur added cost. The SIF recommends providing a few replicates per batch (e.g. 1 replicate per 8-12 samples) with more replicates recommended for more complex matrices (e.g. filters, soils, etc.).
Other helpful tips:
Removing carbonates from calcareous soils and sediments before 13C analysis
Include replicates to check precision, especially for complex matrices