Compound Specific Analysis of 2H and 13C in n-Alkanes
Isotope-Ratio Mass Spectrometry
Straight-chain alkanes, or n-alkanes, are readily measured following extraction from plant, soil, sediment, or petroleum sources [1-3]. CSIA primarily targets the n-alkanes of chain lengths ranging from c-10 to c-40; smaller chain can also be targeted for measurement upon request. Compounds from additional classes of alkanes, such as pristane and phytane may also be measured.
The n-alkanes are dissolved in heptane or pentane, injected at 300 ºC (splitless, 1 min), and separated on an Agilent DB-5ms UI column (60 m x 0.25 mm ID x 1 µm film thickness) at constant flow rate of 1.2 mL/min under the following temperature program: 50 °C (hold 1 min); 120 °C (10 °C/min); 310 °C (5 °C/min; hold 40 min).
GC-C-P-IRMS is performed on a Thermo Trace GC 1310 gas chromatograph coupled to a Thermo Finnigan MAT 253 isotope-ratio mass spectrometer via a GC IsoLink II combustion interface. For 13C analysis, individual n-alkanes are converted to CO2 within a combustion reactor composed of a NiO tube containing CuO/NiO wires maintained at 1000 °C. Water is subsequently removed through a nafion dryer and the analyte gases transferred to the IRMS. For 2H analysis, individual n-alkanes are converted to H2 within a high-temperature thermal conversion reactor of graphitized Al2O3 tube maintained at 1425 °C.
One of every five samples are analyzed in duplicate. Replicates of the quality control and assessment materials are measured every 5 samples.
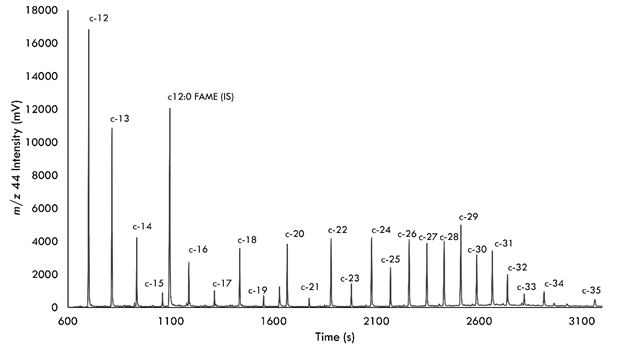
Fig.1. Chromatogram of δ13C analysis of n-alkanes from oils.
UCD SIF n-Alkanes Compound List
Typical analysis includes chain lengths c-10 to c-40. Shorter n-alkanes and compounds from additional classes of alkanes, such as pristane and phytane, may be available upon consultation.
Calibration and Reporting of Stable Isotope Ratios
Quality control and assessment mixtures are composed of pure n-alkanes that have been calibrated separately by EA- and TC/EA-IRMS using certified reference materials (e.g. NBS-22, IAEA-CH-7) distributed by NIST (Gaithersburg, MD U.S.A.) and the USGS (Reston, VA U.S.A.) or are certified mixtures distributed by Indiana University (Bloomington, IN U.S.A.). All are directly traceable to the primary isotopic reference material for each element (δ13C: V-PDB; δ2H: V-SMOW). All calibration procedures for CSIA of n-alkanes are applied identically across reference and sample materials. First, the provisional isotopic value for each n-alkane is obtained by normalization to an isotopically-calibrated internal reference compound (e.g. c12:0 FAME, c13:0 FAME). Isotopic values of the individual n-alkanes are then scale-normalized to the primary reference material (δ13C: V-PDB; δ2H: V-SMOW) using an external mixture composed of n-alkanes with a broad range of calibrated δ13C and δ2H values, IU A7 (or equivalent). Through each calibration step, n-alkanes mixtures and secondary QA materials are monitored for accuracy and precision. Final quality assessment and acceptance of analysis is based on the accuracy and precision of the unbiased quality assessment materials.
Acceptance or rejection of calibrated data is based on the accuracy and precision of an unbiased quality assurance materials, a second δ13C- and δ2H-calibrated n-alkanes mixture, IU B6 (or equivalent). Acceptance of sample measurements require that the final calibrated isotopic values and mean standard deviation (σ) of the quality assurance replicates fall within expected measurement error (δ13C <±0.4‰; δ2H <±6‰). Precision estimates from the co-measured calibrated n-alkanes mixtures and quality assurance materials are provided with data reports.
Measurement Uncertainty
Sample materials are inherently variable in n-alkanes composition. Some n-alkanes may not be measureable and measurement error among n-alkanes varies between different sample types due to differences in composition and chromatographic resolution. Accuracy and precision of the co-measured calibrated n-alkanes mixtures and quality assurance materials are provided with data reports. Limit of quantification, based on total peak area, is generally 1 V-s for δ13C and > 10 V-s for δ2H.
References
[1] M. Bjoroy, K. Hall, P. Gillyon, J. Jumeau. 1991. Carbon isotope variations in n-alkanes and isoprenoids of whole oils. Chemical Geology 93: 13-20. doi: 10.1016/0009-2541(91)90060-5
[2] G. Rieley, R. Collier, D. Jones, G. Eglinton, P. Eakin, A. Fallick. 1991. Sources of sedimentary lipids deduced from stable carbon isotope analyses of individual compounds. Nature: 352: 425-427. doi: 10.1038/352425a0
[3] Y. Chikaraishi, H. Naraoka. 2003. Compound-specific δD-δ13C analyses of n-alkanes extracted from terrestrial and aquatic plants. Phytochemistry 63: 361-371 doi: 10.1016/S0031-9422(02)00749-5